Support
Cancer starts at a molecular level, when multiple mutations within the DNA of a cell cause uncontrolled cellular division without triggering apoptosis, or scheduled cell death. Pharmaceutical companies have developed chemotherapeutics that stop cancer by using molecules called intercalators to target DNA directly. Intercalators are small, planar molecules that force themselves between DNA base pairs, warping the tightly coiled double helix until it breaks. RNA cannot transcribe the broken DNA, which renders the cancerous cell unable to multiply, and slowing the progression or killing the cancer outright. Due to the unique way intercalators work, they are highly effective on many kinds of cancer. However, these drugs can cause a range of side effects, including chronic heart problems. Considering the influential role of intercalators in childhood cancer treatment, developing new intercalator with minimal side effects is an intense area of research.
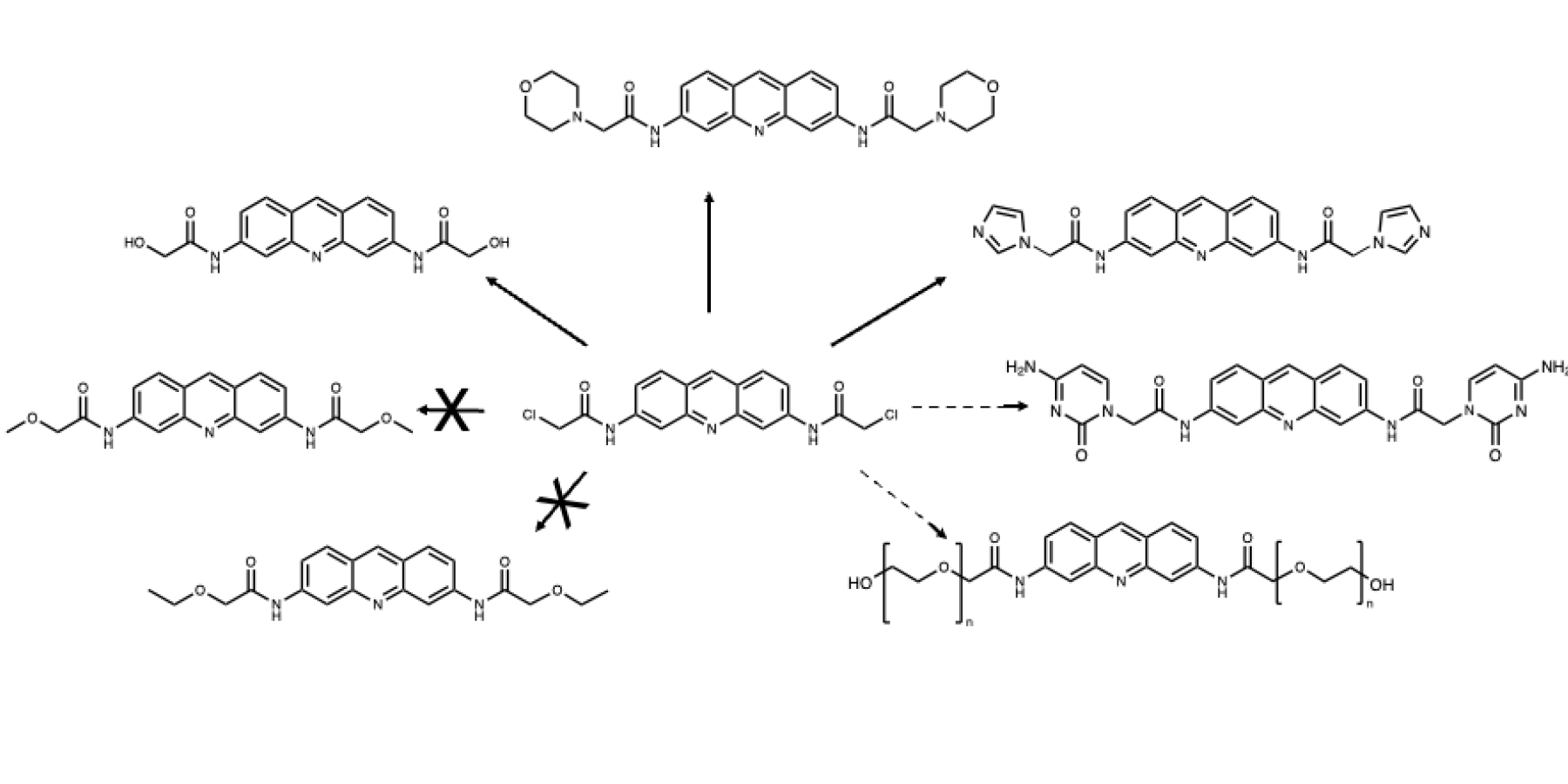
One of the most cited compounds in this field is proflavine, a known intercalator that shows promising anti-cancer properties. Earlier research shows that proflavine derivative are insoluble in water, which makes them biologically irrelevant because they cannot dissolve in the bloodstream. Therefore, I designed a multistep synthesis that would allow me to create an array of novel proflavine derivatives in the hopes that one of the derivatives would be water soluble. The first step in the multistep synthesis involved creating a foundational derivative. While past research cited concerns in the form of complex and time-consuming synthesis, as well as expensive reagents, the foundational derivative synthesis used relatively inexpensive, widely available materials, and was only one step. The foundational derivative was easily modified with small molecules, allowing for the systematic development of novel proflavine derivatives.
During the eight weeks I spent at St. Lawrence this summer, I developed an efficient procedure that produced the foundational derivative in high yield and purity, which is a huge step in creating subsequent derivatives. From the foundational derivative, I synthesized three novel proflavine derivatives, and I hope to synthesis more derivatives in the coming year, focusing on water-solubility. These derivatives will be submitted to a DNA binding study to determine if they’ve retained their intercalative properties.