Support
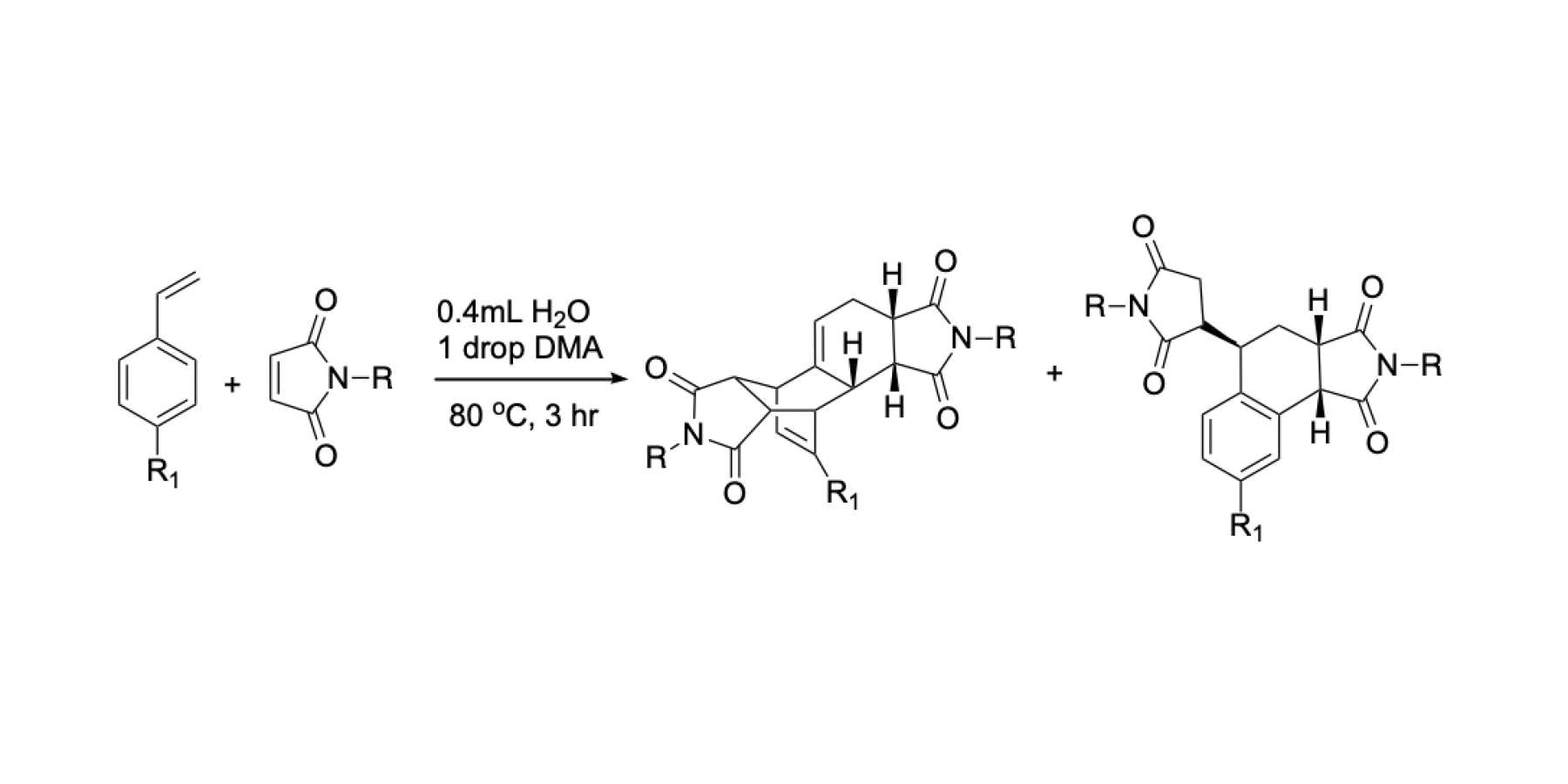
The Wagner-Jauregg reaction is a powerful, yet largely unexplored organic reaction. This reaction pathway is a specialized type of Diels-Alder reaction that facilitates the formation of large, complex ring structures. This reaction is particularly impactful in synthetic chemistry due to the use of inexpensive and readily available starting materials to create more complex molecules. The Wagner-Jauregg reaction yields two primary products: the double Diels-Alder product and the ene product. Both of these products contain complex ring structures at their core that are integral to the synthesis of pharmaceuticals.
The primary goal of this study was to optimize the conditions of the Wagner-Jauregg reaction and to investigate the influence of various substituted styrenes on the overall reaction. In this mechanism, the styrenes served as the diene while the maleimide acted as the dienophile. The initial step of the research focused on identifying the best solvent for the Wagner-Jauregg reaction. Various solevents were screened, and the one providing the highest percent conversion, and the most reproducible results was selected for further experimentation. Under the optimized conditions, a series of substituted styrenes were reacted with the same dienophile. The functional groups on the styrenes affected both the efficiency of the reaction and the products formed during the reaction. These findings contribute to a deeper understanding of the Wagner-Jauregg reaction and highlight its potential utility in the synthesis of complex molecular architectures.